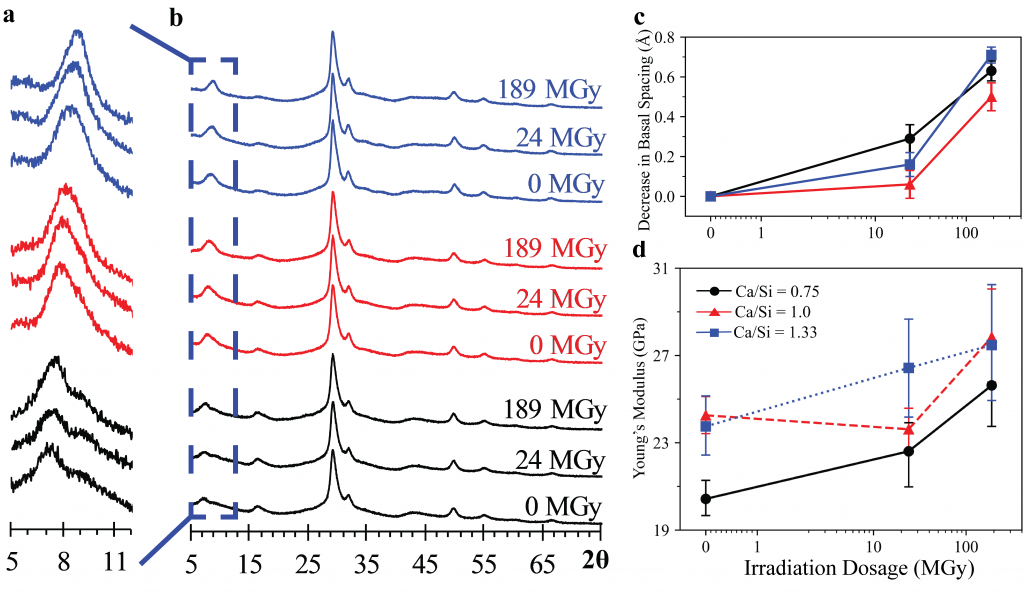
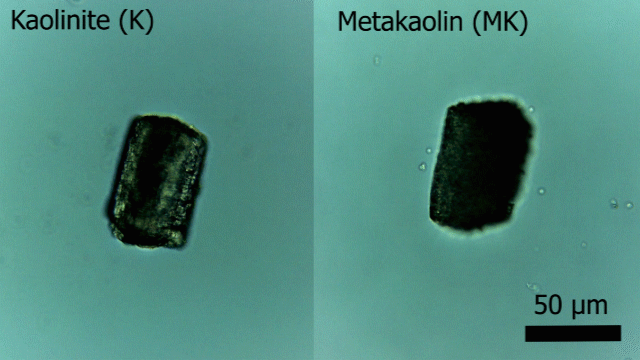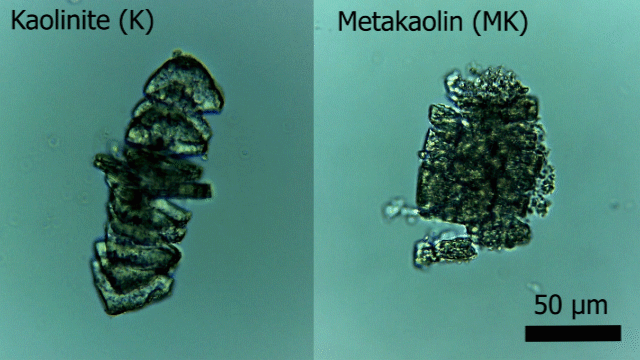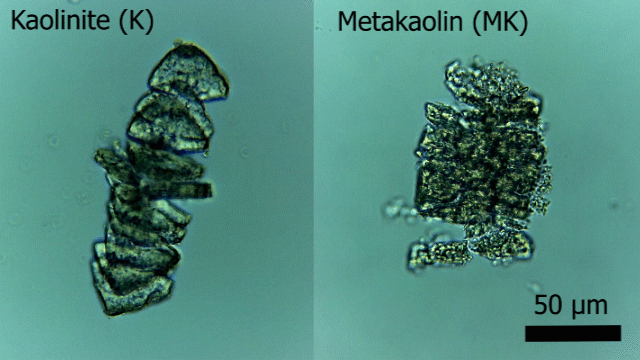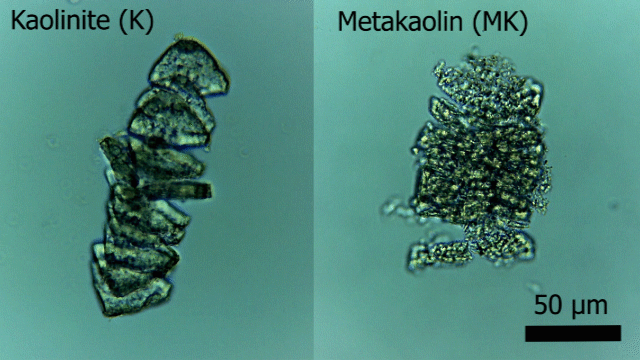
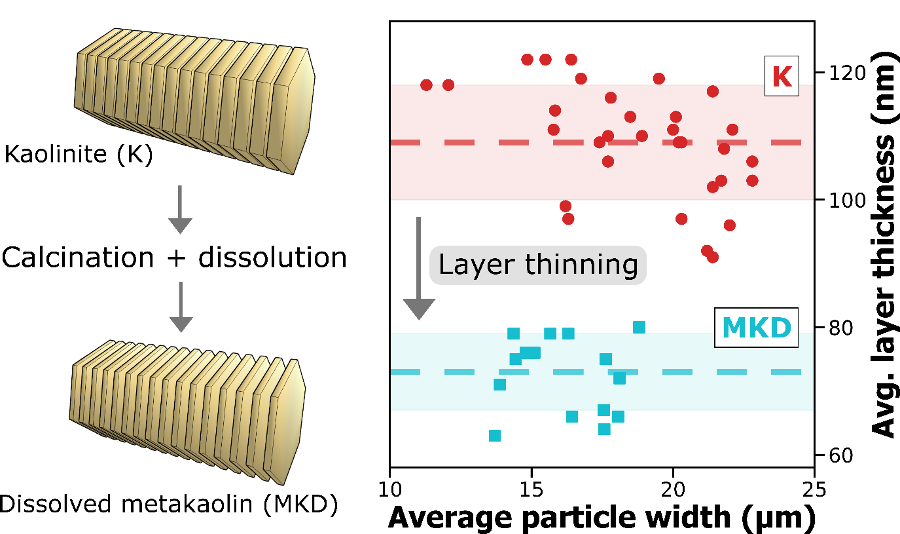
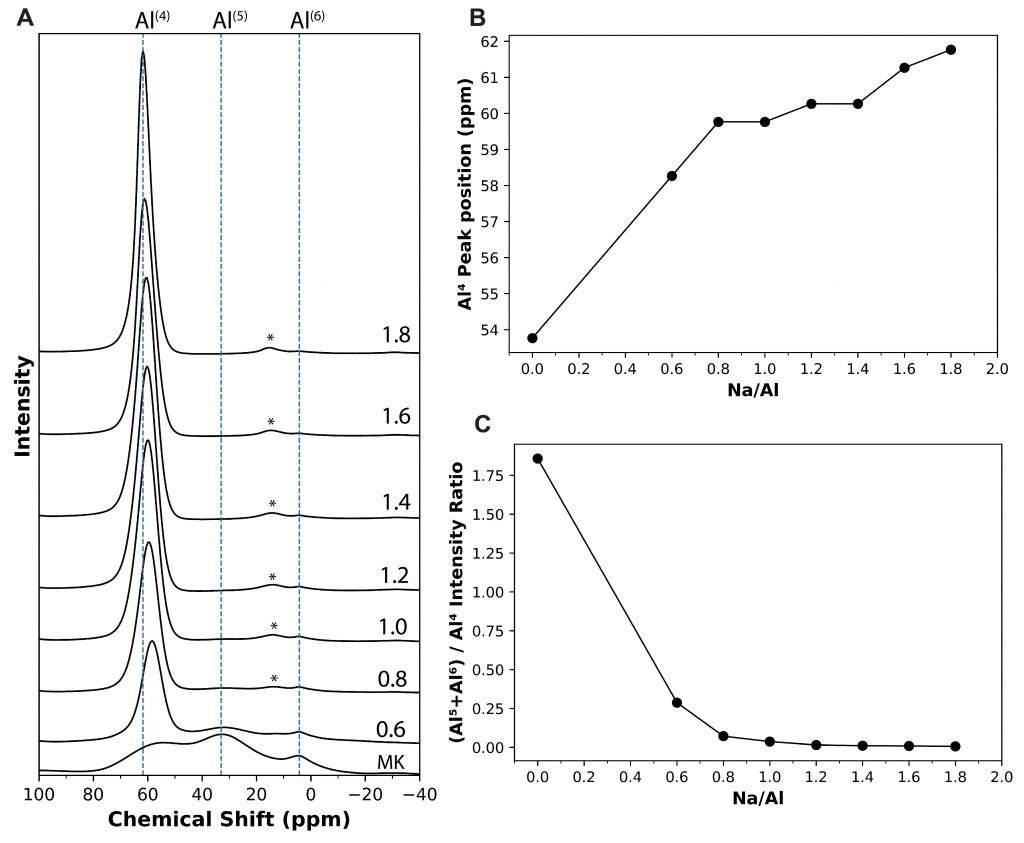
A new publication titled “Superhydrophobic and Self-Cleaning Aluminum via a Rapid and Controlled Process” was published in ACS Applied Engineering Materials in October 2022.
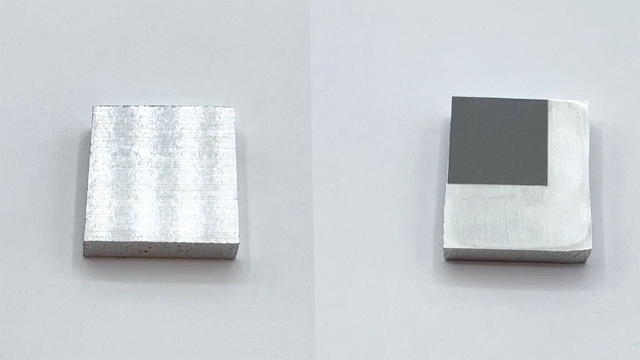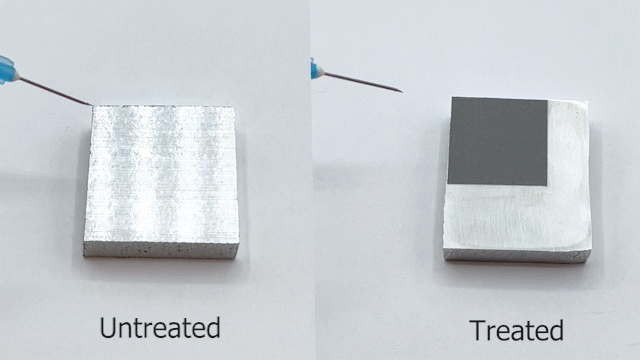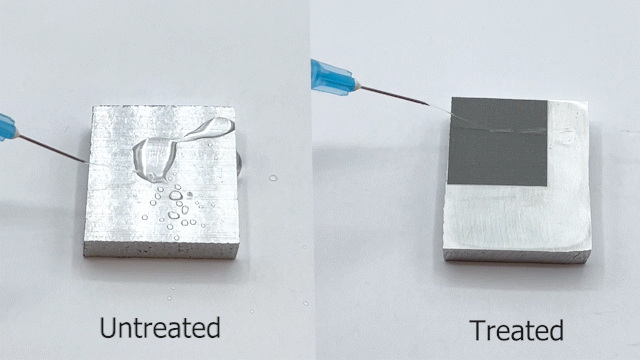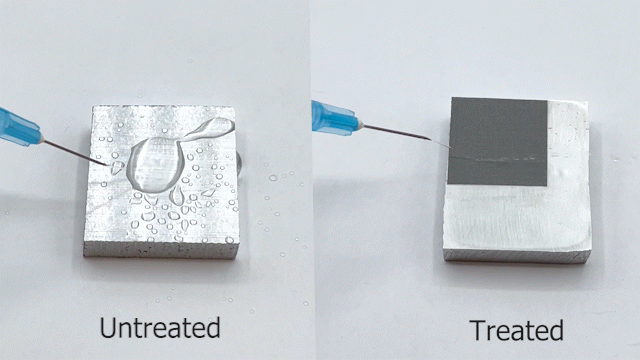
Engineers have been often inspired by nature in various ways, ranging from the design of structures to the selection of materials. For e.g., lotus leaves exhibit hydrophobic behavior which has been translated to the creation of self-cleaning materials. One construction material of interest is aluminum for light-weight structural applications. Previously, studies have reported creation of superhydrophobic aluminum surfaces. However, most of the processes are not environmentally friendly, are time-consuming, and some are not feasible for large-scale applications. In our most recent paper, we present a rapid and controlled process to create superhydrophobic aluminum for use in external environments. With a 1 hr of fabrication time, we achieve contact angle of 158.06° and a sliding angle of 1.94°. Finally, the surface is durable and resilient when exposed to a range of extreme temperatures (-18°C to 100°C). These results pave the way for implementation of superhydrophobic aluminum surfaces for large-scale structural and construction applications.
This is the first article from our group’s MS candidate Ravi Sharma.
Congratulations Ravi!
The article can be accessed here.